

Rheolytic Thrombectomy Device Market Analysis: Global Industry Size, Growth and Forecast
Report ID : 1359423 | Published : August 2025 | Study Period : 2023-2033 | Format : PDF + Excel
The market size of the Rheolytic Thrombectomy Device Market is categorized based on Product Type (Mechanical Thrombectomy Devices, Aspiration Thrombectomy Devices, Combination Devices) and Application (Acute Ischemic Stroke, Peripheral Artery Disease, Coronary Thrombosis) and End-User (Hospitals, Ambulatory Surgical Centers, Specialty Clinics) and geographical regions (North America, Europe, Asia-Pacific, South America, and Middle-East and Africa).
Valued at $400 million in 2023, the Rheolytic Thrombectomy Device Market size is expected to grow to $850 million by 2033, with a CAGR of 8.1% from 2024 to 2033. The report comprises various segments and analyzes the trends and factors playing a substantial role in the market.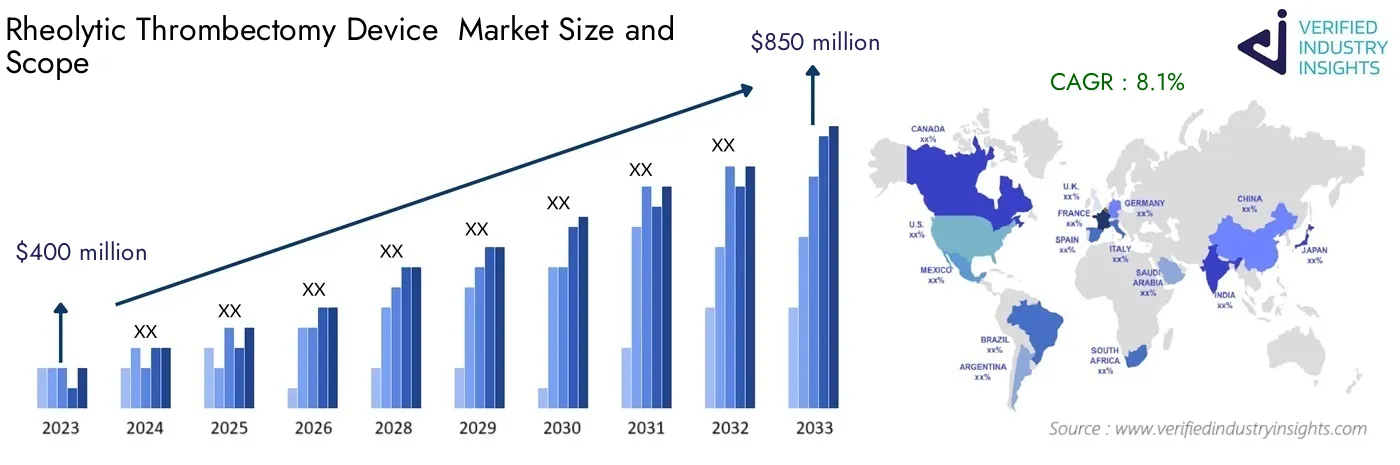
The Rheolytic Thrombectomy Device industry is surely gaining importance in the realm of medical technology as well as in treatment of thromboembolic diseases. Minimally invasive treatment has been a norm across so many countries so the need for cutting edge technology which ensures restoration of blood flow infection-free has been on the rise. Rheolytic thrombus disintegration which involves the two-fold action of drug injection and mechanical force is becoming more and more popular with health care practitioners who want to improve the result of surgery with lesser risks.
In fact, with the combination of a growing elderly population, and the increasing occurrence of diseases like cardiovascular and brain strokes, this need of advanced thrombectomy devices is increased more than ever. It is a fast-moving market with rapid pace in the development of innovative technologies, increased spending on research and development as well as the severe competition of numerous well-known companies and new businesses. Recognizing the features of this market allows all the parties in the market to understand what expenses should be minimized, what goals should be set, and how the healthcare market is going to change.
| ATTRIBUTES | DETAILS |
| STUDY PERIOD | 2023-2033 |
| BASE YEAR | 2023 |
| FORECAST PERIOD | 2024-2033 |
| HISTORICAL PERIOD | 2023-2024 |
| UNIT | VALUE (USD BILLION) |
| KEY COMPANIES PROFILED | Medtronic, Boston Scientific, Terumo Corporation, Penumbra Inc., Stryker Corporation, Abbott Laboratories, AngioDynamics, Cook Medical, Bard Peripheral Vascular, Johnson & Johnson, Cardinal Health |
| SEGMENTS COVERED |
By Product Type - Mechanical Thrombectomy Devices, Aspiration Thrombectomy Devices, Combination Devices
By Application - Acute Ischemic Stroke, Peripheral Artery Disease, Coronary Thrombosis
By End-User - Hospitals, Ambulatory Surgical Centers, Specialty Clinics
By Geography - North America, Europe, APAC, Middle East Asia & Rest of World. |
Rheolytic Thrombectomy Device Market Dynamics
The Rheolytic Thrombectomy Device Market is undergoing significant changes due to various dynamic factors. This section delves into the key drivers, restraints, opportunities, and challenges that are shaping the market landscape.
Market Drivers
- Technological Advancements
- Increasing Consumer Demand
- Regulatory Support
- Globalization
Market Restraints
- High Operational Costs
- Regulatory Challenges
- Market Saturation
Market Opportunities
- Emerging Markets
- Product Innovation
- Strategic Partnerships
Market Challenges
- Technological Disruptions
- Supply Chain Issues
- Changing Consumer Preferences
Rheolytic Thrombectomy Device Market Segmentations
Market Breakup by Product Type
- Overview
- Mechanical Thrombectomy Devices
- Aspiration Thrombectomy Devices
- Combination Devices
Market Breakup by Application
- Overview
- Acute Ischemic Stroke
- Peripheral Artery Disease
- Coronary Thrombosis
Market Breakup by End-User
- Overview
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics
Market Breakup by Region
North America
- United States of America
- Canada
- Mexico
Europe
- United Kingdom
- Germany
- France
- Italy
- Spain
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Key Players in the Rheolytic Thrombectomy Device Market
This report provides an in-depth analysis of both established and rising industry participants. It provides broad lists of important companies organized by the types of products they offer and other market-related factors. In addition to characterizing these companies, the report contains the year each player entered the market, which is useful for research analysis by the study's analysts.
- Medtronic
- Boston Scientific
- Terumo Corporation
- Penumbra Inc.
- Stryker Corporation
- Abbott Laboratories
- AngioDynamics
- Cook Medical
- Bard Peripheral Vascular
- Johnson & Johnson
- Cardinal Health
Customization Options
Verified Industry Insights offers one of the following report customization options to our respectable clients :
Company Profiling
● Detailed profiling of additional market players (up to three players)
● SWOT analysis of key players (up to three players)
Competitive Benchmarking
● Benchmarking of key players on the following parameters : Product portfolio, geographical reach, regional presence, and strategic alliances.
Custom Research
Verified Industry Insights offers custom research services across sectors. In case of any custom research requirement related to market assessment, competitive benchmarking, sourcing and procurement, target screening, and others, please send your inquiry to [email protected] or call us at +1 743 222 5439
© 2024 Verified Industry Insights. All Rights Reserved

